Developmental biology research group
One of the key processes in the animal development is morphogenesis, the process by which the shape of tissues / organs is formed. During development, many morphogenetic events involve rearrangement of simple 2D cell layers into complex 3D tissues. In the last several decades experiments on different model organisms has revealed that certain group of highly conserved signaling molecules are repeatedly utilized in various stages and processes of development. Despite the increasing knowledge of the molecular basis of morphogenesis, how the activity of these signaling pathways is translated into changes in cell behavior and how cell shape changes affect developmental signaling, remain poorly understood. Since morphogenesis involves coordinated dynamic cellular processes (e.g. cytoskeleton remodeling, cell migration, cell shape changes, cell proliferation), tracking morphogenetic events in a spatial-temporal manner is crucial for understanding the mechanisms of development.
Our team uses the developing wing of the Drosophila melanogaster (fruit fly) as a model to elucidate, how the dynamics of tissue morphogenesis and communication between cells are regulated. We expect that our projects will provide novel insights into how dynamics cell-cell communication leads to 3D morphogenesis, and anticipate that our findings will have implications for understanding human pathogenesis of developmental disorders.
Image used in header: The developing wing of the Drosophila melanogaster (author: Osamu Shimmi)
-
Ngan Vi Tran, Martti P Montanari, Jinghua Gui, Dmitri Lubenets, Léa Louise Fischbach, Hanna Antson, Yunxian Huang, Erich Brutus, Yasushi Okada , Yukitaka Ishimoto, Tambet Tõnissoo, Osamu Shimmi (2024). Programmed disassembly of a microtubule-based membrane protrusion network coordinates 3D epithelial morphogenesis in Drosophila. https://doi.org/10.1038/s44318-023-00025-w
-
Antson, H., Huang, Y., Tõnissoo, T., & Shimmi, O. (2023). Conditional knockdown protocol for studying cellular communication using Drosophila melanogaster wing imaginal disc. STAR protocols, 4(4), 102566. Advance online publication.
-
Huang, Yunxian; Gui, Jinghua; Myllymäki, Satu-Marja; Mikkola, Marja L.; Shimmi, Osamu (2023). Coordination of tissue homeostasis and growth by the Scribble-α-Catenin-Septate junction complex. iScience.
-
Toddie-Moore, Daniel J.; Montanari, Martti P.; Tran, Ngan Vi; Brik, Evgeniy M.; Antson, Hanna; Salazar-Ciudad, Isaac; Shimmi, Osamu (2022). Mechano-chemical feedback mediated competition for BMP signalling leads to pattern formation. Developmental Biology, 481, 43−51.
-
Montanari, Martti P.; Tran, Ngan Vi; Shimmi, Osamu (2022). Regulation of spatial distribution of BMP ligands for pattern formation. Developmental Dynamics, 251 (1), 198−212.
-
Antson, Hanna; Tõnissoo, Tambet; Shimmi, Osamu (2022). The developing wing crossvein of Drosophila melanogaster: a fascinating model for signaling and morphogenesis. Fly, 16 (1), 118−127.
-
Huang, Yunxian; Gui, Jinghua; Myllymäki, Satu-Marja; Roy, Kallol; Tõnissoo, Tambet; Mikkola, Marja L.; Shimmi, Osamu (2022). Scribble and α-Catenin cooperatively regulate epithelial homeostasis and growth. Frontiers in Cell and Developmental Biology, 10, 912001.
-
Gui, J., Huang, Y., Montanari, M., Toddie-Moore, D., Kikushima, K., Nix, S., Ishimoto, Y. and Shimmi, O. (2019). Coupling between dynamic 3D tissue architecture and BMP morphogen signaling during Drosophila wing morphogenesis. Proc. Natl. Acad. Sci. U.S.A. 116, 4352-4361
- 2022. a. juunikuu Eesti Looduse numbris jätkasid TÜMRI teadlased Osamu Shimmi, Tambet Tõnissoo ja Sulev Kuuse eelmises kuus ilmunud artiklit, kus anti ülevaade biokuvamisest. Seekord kirjutasid teadlased, kuidas saab jälgida molekule ja rakuorganelle ning mil moel võivad uued meetodid olla kasulikud meditsiinis.
- 2022. a. maikuu Eesti Looduse numbris avaldasid TÜMRI teadlased Osamu Shimmi, Tambet Tõnissoo ja Sulev Kuuse artikli, kus teemaks on põnev tehnoloogia: biokuvamine.
-
TÜMRI arengubioloogia õppetooli juhataja, kaasprofessor Tambet Tõnissoo, kirjutab ajakirjas Eesti Loodus teemal: „Kuhu küll kõik karvad jäid?“ Eesti Loodus, 03.2022
- Ajakirja Eesti Loodus 2021. a. detsembrinumbris ilmus TÜMRI arengubioloogia õppetooli juhataja ja arengubioloogia kaasprofessori Tambet Tõnissoo sulest põnev artikkel, milles ta kommenteerib kalifornia kondorite partenogeneesi.
- Ajakirja Eesti Loodus 2021.a. märtsinumbris vestleb Tartu Ülikooli arengubioloogia professori Osamu Shimmiga arengubioloogia kaasprofessor Tambet Tõnissoo.
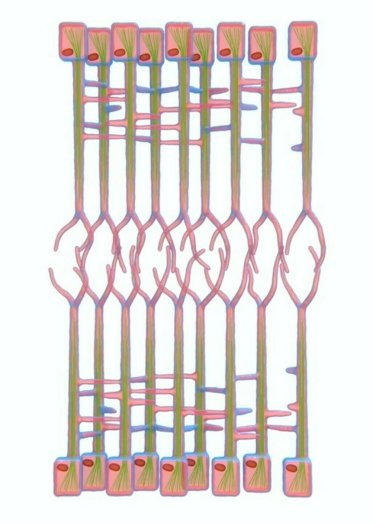
To generate complex 3D organs, the shape of each cell needs to change to support the overall development of the tissue. Since these shapes are always changing, it is important to establish a protocol to monitor these changes as they happen in real time. In the developing wing of a fruit fly, Drosophila melanogaster, there are two layers of cells that are nearly identical and lie opposite each other. This study used live imaging to observe a network of cells that exists between these two layers, called the Interplanar Amida Network (IPAN). When this network loses its cell-to-cell contacts, it affects cell division in both layers, which is necessary for 3D tissue growth. This finding suggests that IPAN contributes to coordinating how tissues develop into 3D structures by changing cell shapes.
Image: Interplanar Amida Network (IPAN) (amida = ghost leg) Schematic of Interplanar Amida Network (IPAN)